The number of biomaterials available is rising every year in the worldwide dental market. For dental professionals this is good news, because competition between market leaders can bring new materials and technologies as well as the development of the existing ones.
In this article, I will share my knowledge about the handling and working of a system of biomaterials from Botiss. The case presented is only a sample of the full documentation, but I believe it gives an indication of the way we work with selected biomaterials. I was impressed with the full range of products and complete system, which gives the choice to select the optimum biomaterial type in every single case.
An interesting product is bovine origin xenograft material Cerabone, which is on the German market with approval from the Health Ministry that it is 100 per cent BSE safe. With a closer look at the production of this material we can easily get an answer to this certification. According to the World Health Organization bovine biomaterials are free of prions and other proteins when they are treated at high temperatures (above 800 degrees Celsius) and Cerabone is heated at over 1,200 degrees Celsius in the final stage of production.
Another interesting option for guided bone regeneration (GBR) is a human originated material called Maxgraft. This allograft is available in blocks (cortico-spongious and spongious) and as granulates as well. It’s an interesting alternative for harvesting autogenous bone in some cases. Bone for this material is collected from living donor patients who are accepted for hip joint replacement. After they have undergone very strict and controlled EU regulation tests and cleaning procedures the bone is used to create the material Maxgraft.
With the development of new technologies and techniques, companies can offer clinicians interesting options based on the allografts: one is Maxgraft bonering and the second is Bonebuilder.
The bonering technique was originally developed by Bernd Giesenhagen from Germany.
Initially, the technique was using the autogenous bone of the patient, but in cooperation with Orcan Yuksel they developed the technique in a new level with an introduction of prefabricated bone rings made from allograft.
Bonebuilder is not the only biomaterial name; it is the name of technology. Now we can order individually prepared (shaped) allograft block for a single patient. Precision is amazing and a fit you can compare to Lego blocks. This is possible by a combination of two technologies: CBCT and CAD/CAM.
Guided regeneration is directly combined with different materials based on collagen, starting from collagen sponges that end up as sophisticated barrier membranes.
We can choose from classic collagen membrane like Collprotect or very stable and tearing resistant pericardium like Jason membrane. On top of this is Mucoderm collagen matrix membrane, with properties and structure which gives an option for replacement of subepithelial connective tissue grafts.
It can be used for classic and tunnel techniques as well.
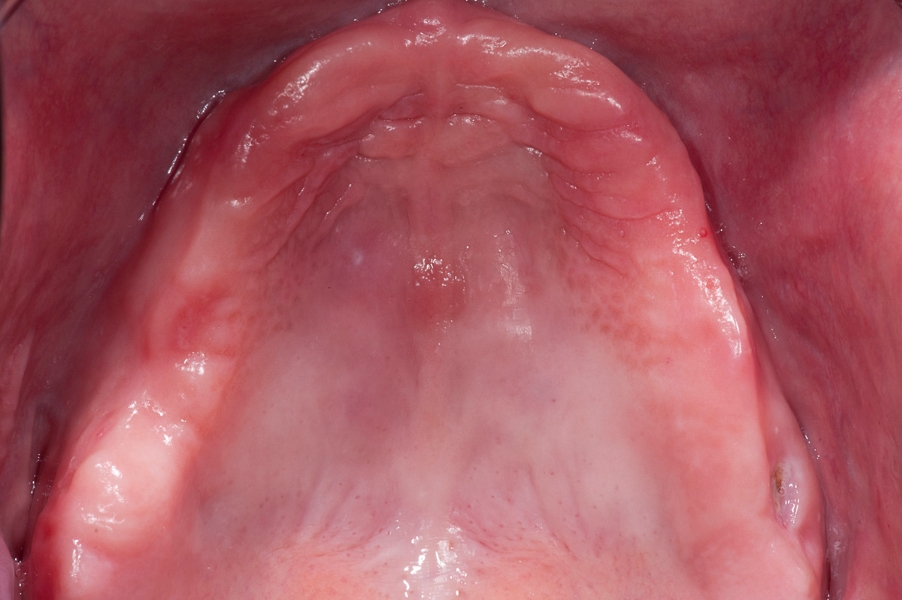
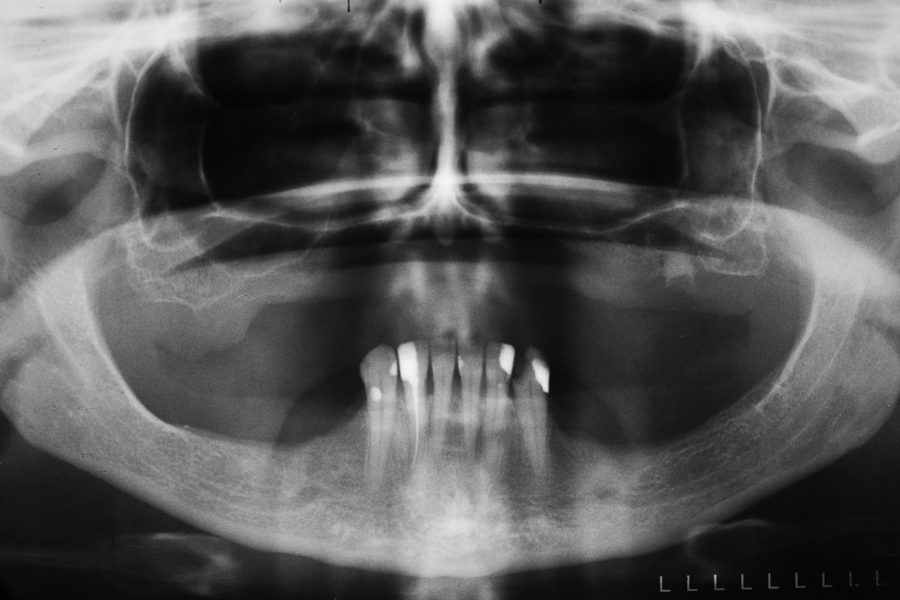
Fig 1: Initial situation – severe resorption Fig 2: Initial panoramix X-ray with high atrophy
of the maxillary ridge. of maxillary ridge and vertical deficiency in
the posterior region.

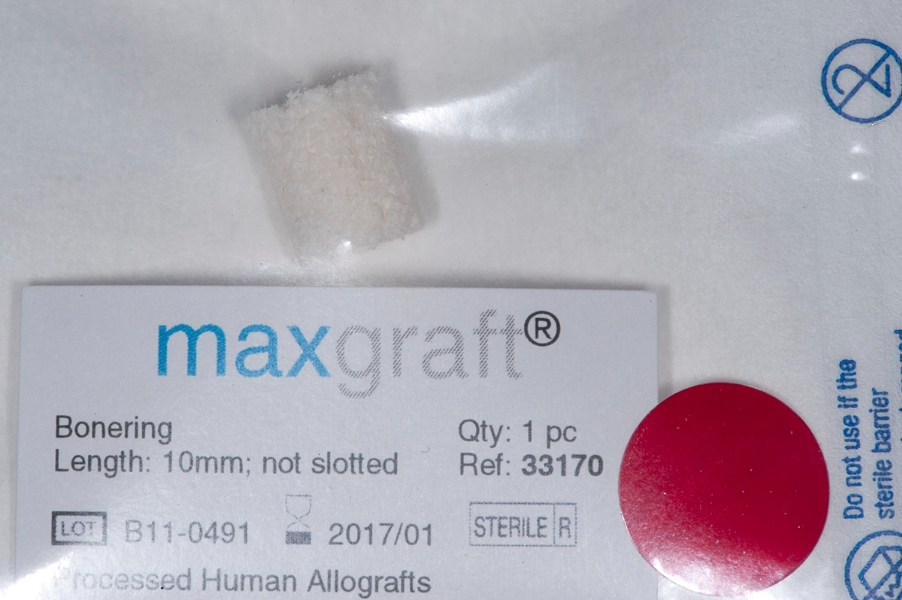
Fig 3: Sinus floor elevation. Less than 2mm Fig 4: Package of Maxgraft bonering. Length:
of bone is left in vertical dimension. 10mm.
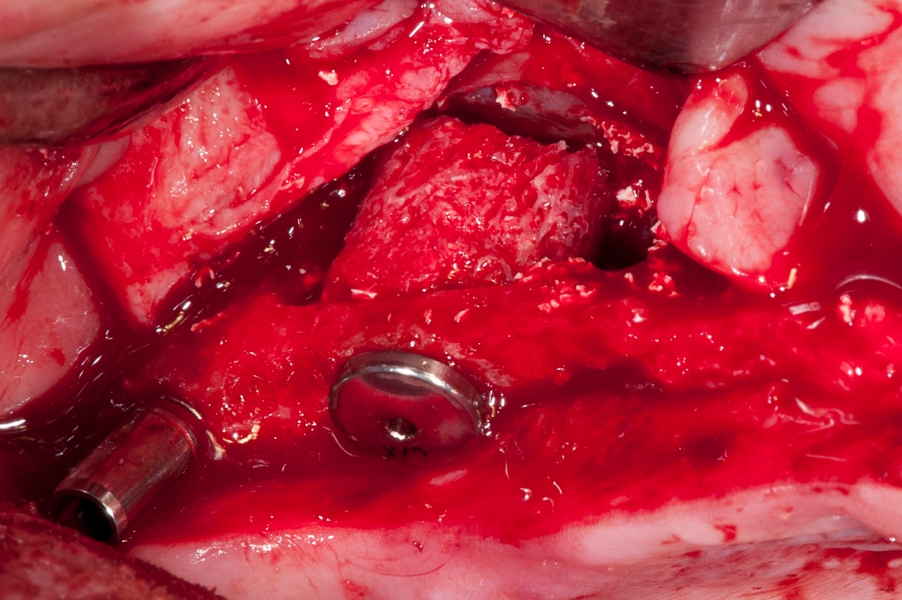
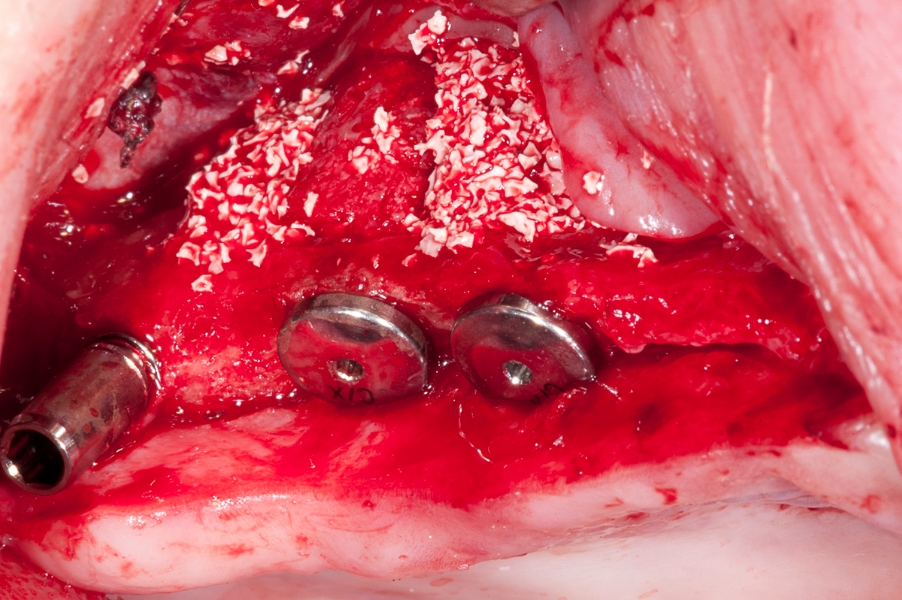
Fig 5: Fixation of the implant with the Fig 6: Three implants in the sinus with space
the Maxgraft bonering in the sinus cavity. in between the Maxgraft rings filled with
Additional special mounting screw is visible. Cerabone - xenograft.
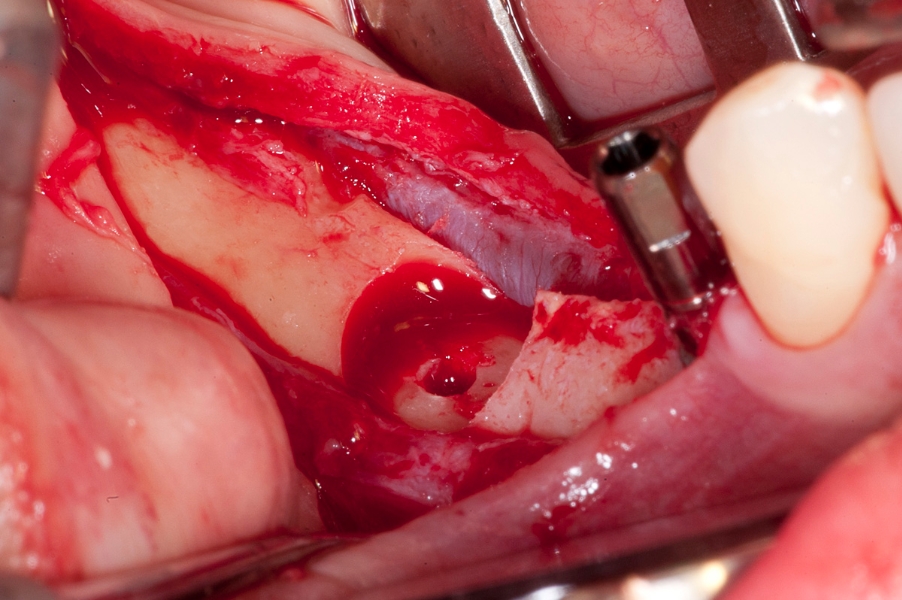
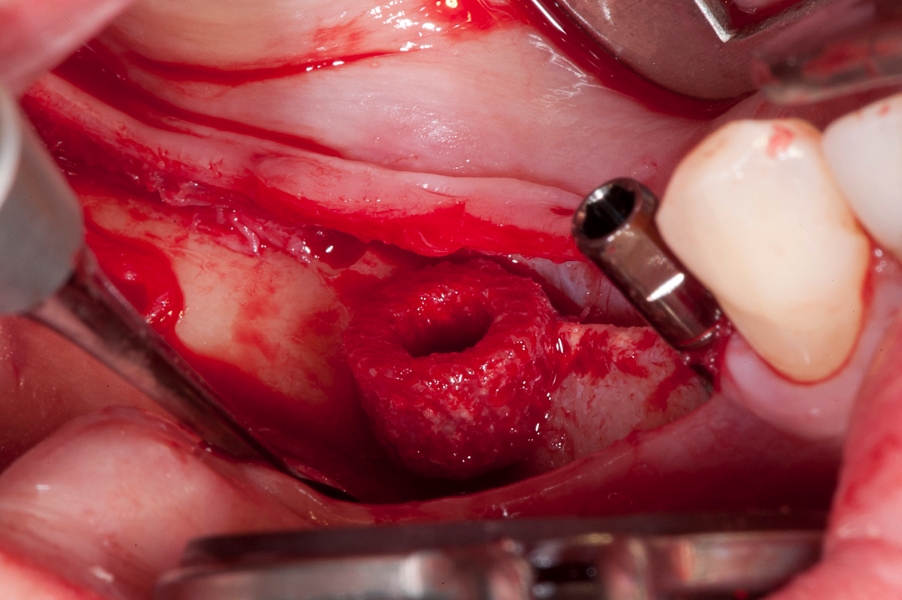
Fig 7: Recipient site preparation for vertical Fig 8: Maxgraft bonering in place.
bone augmentation with bonerings in the
mandible.
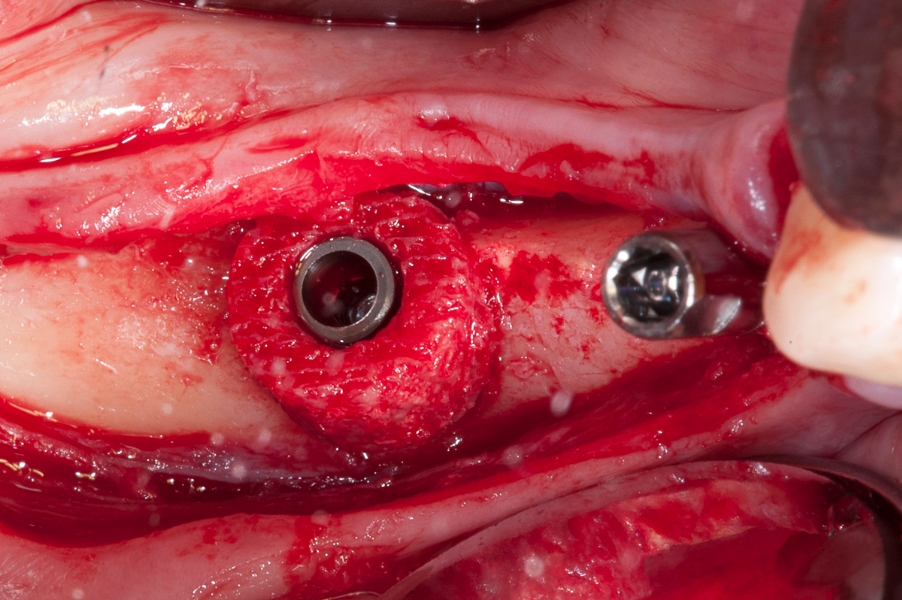
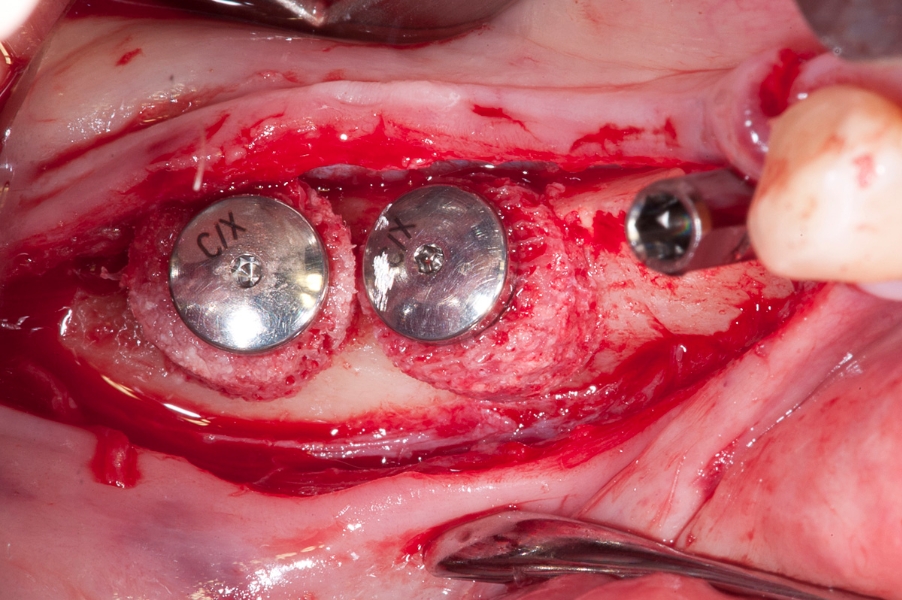
Fig 9: Fixation of the implant inside of the Fig 10: After positioning of two implants with
Maxgraft bonering. Maxgraft bonerings the special fixing screws
are mounted on top of the implants to stabilize
position of the bonerings.

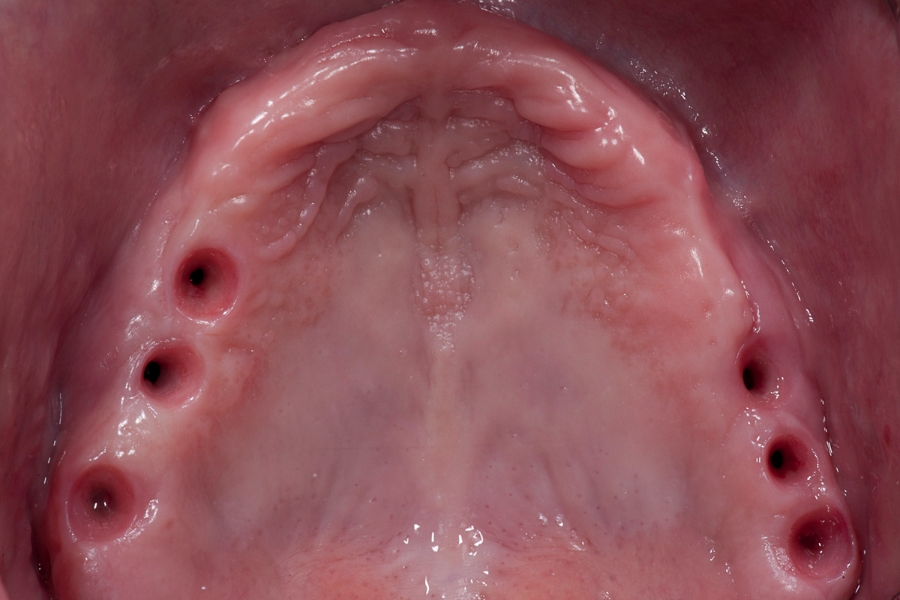
Fig 11: Covering of the augmented site with Fig 12: Situation in the maxilla after implants
xenograft material: Cerabone and collagen opening and healing of the soft tissue.
pericardium Jason membrane. Stabilisation
with titanium pins.
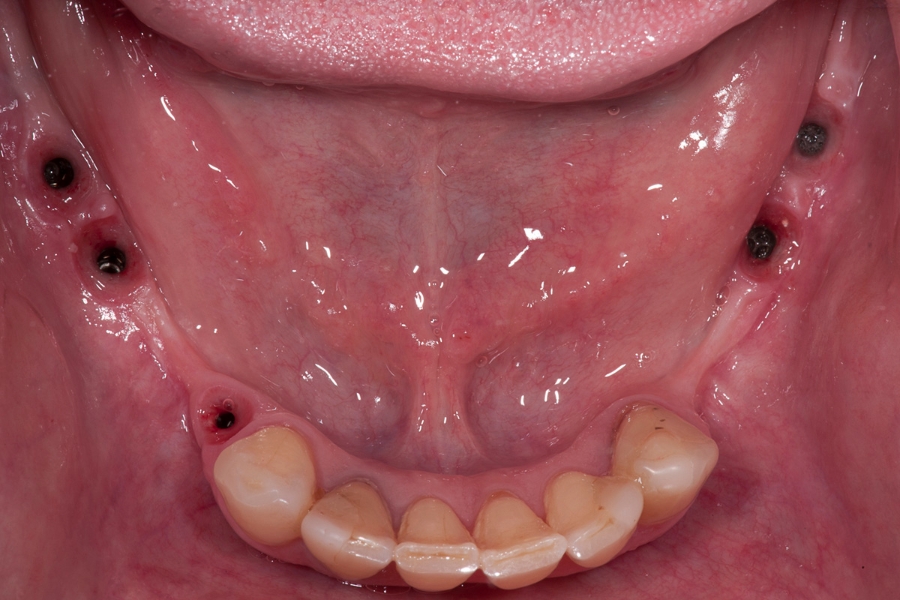
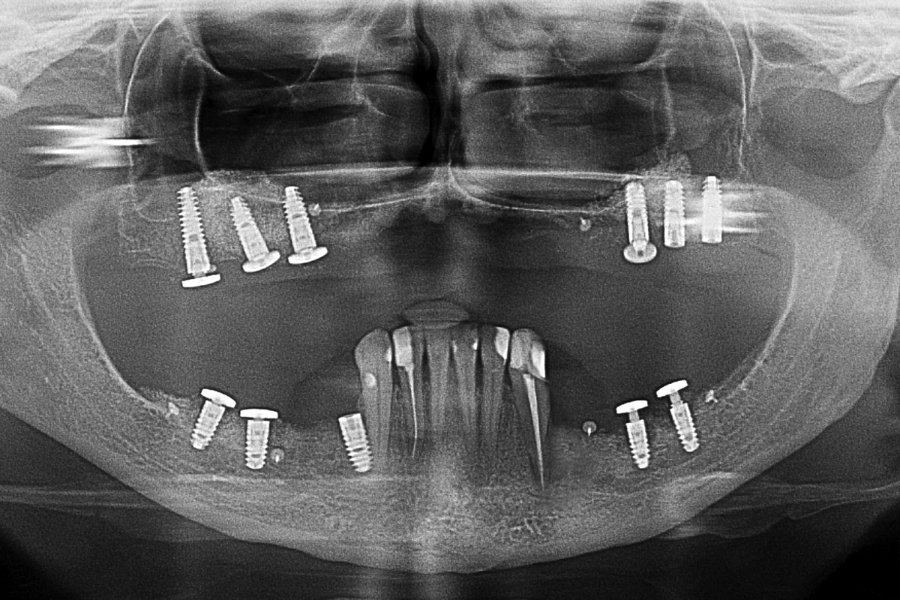
Fig 13: Situation in the mandible after Fig 14: OPG X-ray after surgical procedures.
implants opening and healing of the soft
tissue.
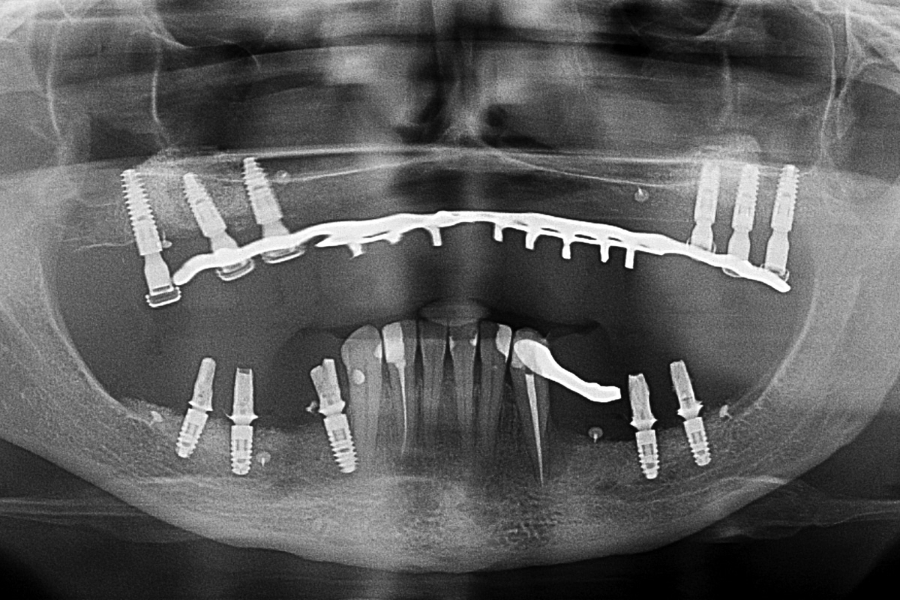
Fig 15: OPG X-ray 12 months later with
temporary restorations.
